What does the atomic number of an element tell you?
- The Atomic Number Of An Element Is The Number Of Protons
- What Is An Atomic Number
- The Atomic Number Of Element Is The Number Of
- Atomic Numbers Of Elements
- Atomic Number Mass Number Element Symbol

1 Answer
- Atomic number definition is - an experimentally determined number characteristic of a chemical element that represents the number of protons in the nucleus which in a neutral atom equals the number of electrons outside the nucleus and that determines the place of the element in the periodic table.
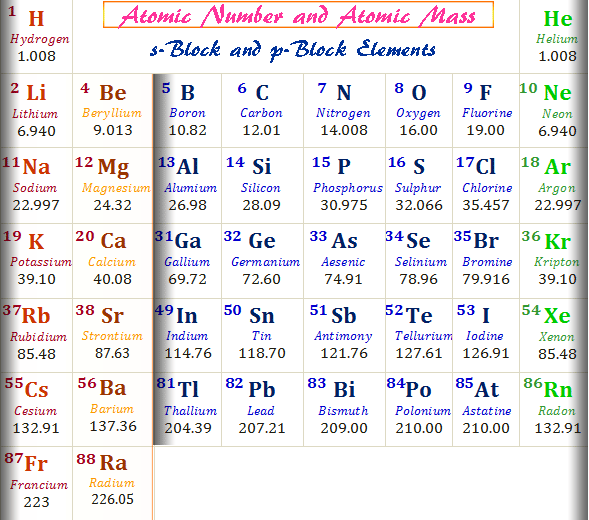
- Here is a list of the elements sorted by atomic number.Element nameElement symbolAtomic numberHydrogenH1HeliumHe2LithiumLi3.
The Atomic Number Of An Element Is The Number Of Protons

The atomic number of an atom represents the number of protons found in the nucleus of that atom. Elements are identified by their atomic numbers because each element has a different number of protons in its nucleus. The periodic table of the elements is organized by atomic numbers.
Identifies the number of protons a single atom of the element contains.
Explanation:
What Is An Atomic Number
The atomic number helps people identify elements according to the number of protons one atom of the element has. It essentially defines the element.
The Atomic Number Of Element Is The Number Of
While having a neutral charge, it also provides the number of electrons the element has (in one atom).
While isotopes are a thing, it doesn't completely change the atom.
Have a different number of neutrons, you have an isotope, but if the number of protons differ, you are dealing with an entirely different element (in neutral state - reactions are out of the picture).
Atomic Numbers Of Elements
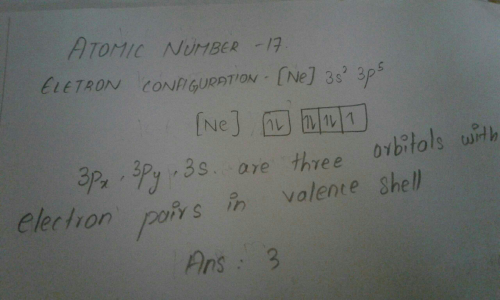
Hope this helps :)
Atomic Number Mass Number Element Symbol
Related questions
