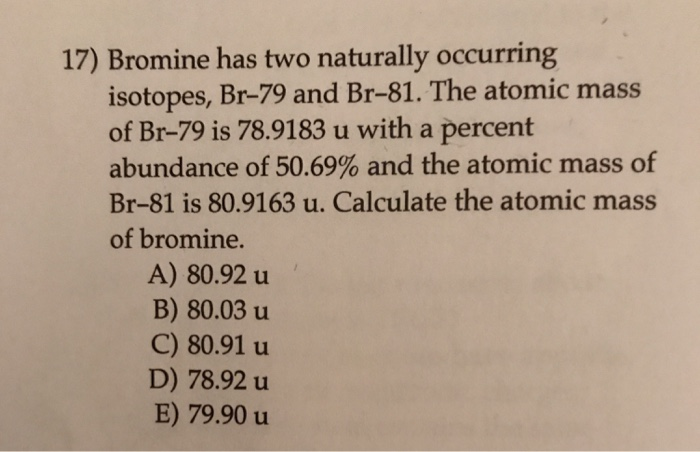
The element bromine, Br (atomic number 35), has two major isotopes of similar abundance, both around 50 percent. The atomic mass of bromine is reported in the periodic table as 79.904 atomic mass units. Choose the most likely set of mass numbers for these two bromine isotopes. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Br-81 (Bromine. Chemical elements listed by atomic mass The elements of the periodic table sorted by atomic mass. Click on any element's name for further information on chemical properties, environmental data or health effects. This list contains the 118 elements of chemistry. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Br-71 (Bromine, atomic number Z = 35, mass number A = 71).
Dobereiners’s Triads
Johann Wolfgang Dobereiner was a German chemist. His effort is considered as one of the earliest attempts to classify the elements into groups.
The earliest classification categorized elements into metals and non-metals. It was difficult to classify the elements, such as boron, which exhibited the properties of both metals as well as non-metals. After further research a German scientist, Dobereiner arrived at a hypothesis in the year 1829
He found that when elements are arranged into groups of three in the order of their increasing atomic mass, the atomic mass of the element; which comes in the middle; is the arithmetic mean of rest of the two. On this basis, he arranged three elements in one group which is known as ‘Triad’. This arrangement of elements is known as Dobereiner’s Triads.
Dobereiner’s Triads | ||
| Elements and their Atomic Mass | ||
| Lithium (Li) 7.0 | Sodium (Na) 23.0 | Potassium (K) 39.0 |
| Calcium (Ca) 40.0 | Strontium (Sr) 87.5 | Barium (Ba) 137.0 |
| Chlorine (Cl) 35.0 | Bromine (Br) 80.0 | Iodine (I) 127.0 |
In this table, atomic mass of sodium is equal to arithmetic mean of atomic masses of lithium and potassium. Similarly, atomic mass of strontium is equal to arithmetic mean of atomic masses of calcium and barium.

Limitation of Dobereiner’s Triads:
Dobereiner could find only three such triads (group of three elements) and he could not even put all the elements known at that time in his triads.
The rules of Dobereiner’s triads could not be applied to the elements which had very low or high atomic mass. Such as; if F, Cl and Br are put together in a triad, in increasing order of their atomic masses, the atomic mass of Cl is not an arithmetic mean of atomic masses of F and Br.
After the advancement of techniques of measuring atomic mass more correctly Dobereiner’s Law became obsolete.
Newlands’ Law of Octaves:
Newlands found that every eighth element has similar physical and chemical properties when they are arranged in increasing order of their relative masses. This law is known as Newlands’ Law of Octaves which states that “any given element will exhibit analogues behavior to the eighth element following it in the table”. This means every eight element has the similar chemical and physical properties. For example; Sodium is the eighth element from Lithium and both have similar properties.
The arrangement of elements in Newlands’ Octave resembles the musical notes. In musical notes, every eighth note produces similar sound. Because of this; Newland’s classification of elements was popularly known as just Octaves.
Limitation of Newlands’ Octaves:
- Newlands’ Octaves could be valid upto calcium only; as beyond calcium, elements do not obey the rules of Octaves.
- Newlands’ Octaves was valid for lighter elements only.
- It appears that Newlands did not expect the discovery of more elements than 56 which were discovered till his time.
- More than one element had to be placed in some of the groups; in order to place the elements having similar properties in one group. But in order to do so, he also put some dissimilar elements in same group.
- Iron; which has similar property as cobalt and nickel, was placed far from them.
- Cobalt and nickel were placed in the group with chlorine and fluorine in spite of having different properties.
- In spite of above limitations; Newlands was the first scientist who arranged the elements in order of their increasing relative atomic masses.
| Chapter 13: Spectroscopy |
Isotope patterns for -Cl and -Br
- Mass spectrometers are capable of separating and detecting individual ions even those that differ only by a single atomic mass unit (note in rality they are far more sensitive than that!)
- As a result, molecules containing different isotopes can be distinguished.
- This is most apparent (at this level) when atoms such as bromine or chlorine are present in a molecule because those elements naturally exist with a significant % of the heavier isotope.
- For example, while C has 2 common isotopes, 12C and 13C, 13C represents only about 1% of natural carbon. In contrast, Cl has 2 common isotopes, 35Cl and 37Cl, with about 25% being 37Cl.
- Typically, one looks at the molecular ion peak, 'M' (since this is being identified and used to determine the MW).
- When working with MW from the molecular ion in MS, the best approach is to always use the lighter ion (i.e. M) and the mass of the lighter isotope (i.e. for Cl use 35 not 35.5, or, for Br use 79 and not 80)
- 35Cl : 37Cl exists naturally in an almost 3:1 ratio, so we observe peaks at 'M' (molecules with an atom of 35Cl) and 'M+2' (molecules an atom of 37Cl) are obtained with relative intensity 3:1
Atomic Mass Of Br 81
- 79Br : 81Br exists naturally in an almost 1:1 ratio, so we observe peaks at 'M' (molecules with an atom of 79Br) and 'M+2' (molecules an atom of 81Br) are obtained with relative intensity 1:1
- Note that since the relative natural abundances of the isotopes are different, you can tell the difference between the presence of Cl and Br. The patterns are different, they look different.
- 'M+1' peaks are usually seen due to the presence of 13C in the sample but because 13C is only about 1% of natural carbon, the peaks tend to be small (unless there is a large number of C atoms present). Note that you can see the small peaks due to the presence of 13C in the figures shown for Cl and Br, they look like little shadows on the right of the other peaks.
The following two examples of mono-haloalkanes mass spectra show the characteristic isotope patterns of monohalogenated molecules. The patterns are highlighted in the green boxes:
Example 1 :
This MS is of 2-chloropropane, C3H7Cl.
Note the characteristic isotope pattern at 78 (M) and 80 (M+2) in a 3:1 ratio.
Loss of 35Cl from 78 or37Cl from 80 gives the base peak a m/z = 43 (M - 35 = M+2 - 37 = 43) corresponding to the secondary propyl cation.
Note that the peaks at m/z = 63 and 65 represent fragment ions that still contain Cl and therefore also show the 3:1 isotope pattern.
The very small peak at 79 represents M+1, the small number of molecules that contain 35Cl and an atom of 13C rather than 12C.
The even smaller peak at 81 presents M+2+1 = M+3, a very small number of molecules that contain 37Cl and an atom of 13C rather than 12C.

Example 2 :
This MS is of 1-bromopropane, C3H7Br.
Note the isotope pattern at 122 and 124 represents the M and M+2 in a 1:1 ratio.
Loss of 79Br from 122 or 81Br from 124 gives the base peak a m/z = 43, corresponding to the propyl cation.
Note that other peaks, such as those at m/z = 107 and 109 (yes, they are small) still contain Br and therefore still show the 1:1 isotope pattern.
Note: the isotope patterns for polyhalogenated molecules (such as having both -Cl and -Br or with multiple -Cl or -Br) give different (but still characteristic isotope patterns).
Br Atomic Mass Number
| © Dr. Ian Hunt, Department of Chemistry |


